常態(tài)形式下的金元素是惰性的����,但在納米形態(tài)時,金元素就會呈現(xiàn)獨特的優(yōu)異催化活性���。納米金原子簇團負載在氧化物上所產生的新的多相催化行為將會對諸多領域產生重大影響���,例如大氣污染物的清除�����、燃料電池用氫氣的生產凈化、精細化學品的制成及有機物的液相氧化反應等;尤其是納米金催化劑的低溫活性����,能為制備常溫環(huán)境的凈化材料作出突出貢獻���。
納米金催化劑是一種新型的催化材料����,其應用涉及眾多反應�����,如催化CO氧化�����、臭氧分解�、水氣轉移反應�����、NOx還原、乙炔氫氯化����、丙烯環(huán)氧化、1,2-二醇選擇性液相氧化等��。納米金催化劑在常溫環(huán)境和濕度下可催化CO����,且能達到完全轉化,因此它可成為軍事和民事生命保障系統(tǒng)中凈化CO的完美選擇��。
美國STREM工廠提供各種高純度��、品質卓越的納米金催化劑���,可滿足從事新材料以及納米催化劑研究的科技人員研究需求����。
2008 年��,百靈威與國際知名品牌STREM 正式簽署戰(zhàn)略合作協(xié)議����,全權負責STREM 在中國市場的產品銷售���、技術應用與支持等各項業(yè)務。百靈威始終秉承“資源共享����,共同發(fā)展”之理念,一如既往地為中國化學行業(yè)廣大科研和生產用戶提供品質卓越的化學相關產品及服務�,為實現(xiàn)“促進科技與工業(yè)發(fā)展,造福人類”的使命而不懈努力!
|
metals • inorganics • organometallics • catalysts • ligands • customsynthesis • cGMP facilities • nanomaterials
|
|
Catalog Number and Name
|
79-0160
Gold 1% on aluminum oxide extrudates
(AUROlite™ Au/Al 2O 3 )
|
79-0165
Gold 1% on titanium dioxide
extrudates
(AUROlite™ Au/TiO 2)
|
79-0170
Gold 1% on zinc oxide granulate
(AUROlite™ Au/ZnO)
|
|
Color and Form
|
dark purple extrudates
~1.2mm dia. x 5mm (avg)
|
dark purple/gray extrudates
1.5mm dia. x 5mm (avg)
|
dark purple granulate
1-2mm dia.
|
|
Analysis
|
Au 1 wt% ± 0.1%
Al2O3 98 wt%
Na+, Cl- <1500ppm
|
Au 1 wt% ± 0.1%
TiO2 98 wt%
Na+, Cl- <1500ppm
|
Au 1wt% ± 0.1%
ZnO 88wt%
(contains Al2O3)
Na+, Cl- <1500ppm
|
|
Other
|
Bulk density: 0.6–0.8 g/ml
Specific surface area:
200-260 m2/g (store cold)
|
Bulk density: 0.85–0.95 g/ml
BET (surface area):
40-50 m2/g (store cold)
|
Bulk density: 1-1.2 g/ml
BET (surface area):
40-50 m2/g (store cold)
Note:PCT WO2005115612
|
|
Available sizes: 10g, 50g
|
|
Sold in collaboration with Project AuTEK for research purposes. Reverse engineering and product modification prohibited.
Only open before use, store cold in dark.
|
|
Technical Note:
1. Useful product for the catalytic oxidation of a variety of substrates including carbon monoxide, aldehydes, alkenes and methane. Average gold crystallite size is ~2-3nm.
References:
1. J.Catal.,2007,252, 119.
2. J.Catal.,2008,260, 86.
3. Green Chem.,2008,10, 168.
4. Gold Bulletin,2008,41, 296.
|
|
"Please note: every care has been taken when producing the gold catalysts and their data sheets, however, users of the catalysts are advised that Mintek and its partners in Project AuTEK bear no responsibility for the performance of the catalysts or any accident, injury, death and / or damage arising from their delivery, handling or use.”
|
Reproduced from The Strem Chemiker, May, 2009.
Capabilities of AUROliteTMCatalysts
Jason S. McPherson and David T. Thompson
Project AuTEK, Advanced Materials Division, Mintek, Private Bag X3015, Randburg 2125, Republic of South Africa
AUROliteTMcatalysts (1wt%gold on titania, alumina and zinc oxide supports) are made in kilogram quantities by Project AuTEK and their advantages over other precious metal catalysts are being demonstrated by achieving high activities and selectivities in both liquid- and gas-phase reactions which have commercial potential.
1. Introduction
Gold catalysts are very active under the mild conditions which favour their selectivity and the economics of the processes involved. Careful choice of preparative conditions and supports helps to maximize their selectivity and activity [1]. Their potential in chemicalprocessing reactions has been clearly demonstrated and there is definite evidence of increasing R&D involving gold and gold-platinum group metal (PGM) combinations [2]. This is likely to result in new industrial applications for gold catalysts in chemical processing and pollution control. Selective oxidation of carbon monoxide in the hydrogen streams used for fuel cells has been achieved using AuTEK catalysts, as is the use of this ambient temperature oxidation process for use in gas masks for protection from CO poisoning and for CO removal from room atmospheres [2,3]. AuTEK has produced three gold catalyst systems, and use of these catalysts, and other closely related gold catalysts to optimize requirements for particular reactions where high activity or selectivity to desirable products is required, is indicated below.
2. Gas Phase Reactions
AUROliteTM catalysts have been evaluated for their CO oxidation characteristics at Oak Ridge National Laboratories, USA [4]. The Au/TiO2 catalyst gave 100% conversion of CO at < O°C and the Au/Al2O3 catalyst was also very active at low temperatures. All three AUROliteTM catalysts are being developed by Project AuTEK for CO oxidation in respiratory protection devices [5]. As can be seen in Figure 1, under typical EN403 (fire escape mask) test conditions the Au/TiO2 is more active and durable than the established commercial technology namely Hopcalite (CuMnOx). Furthermore under these conditions the activity of this material is amplified by the presence of moisture, unlike Hopcalite which experiences rapid deactivation.
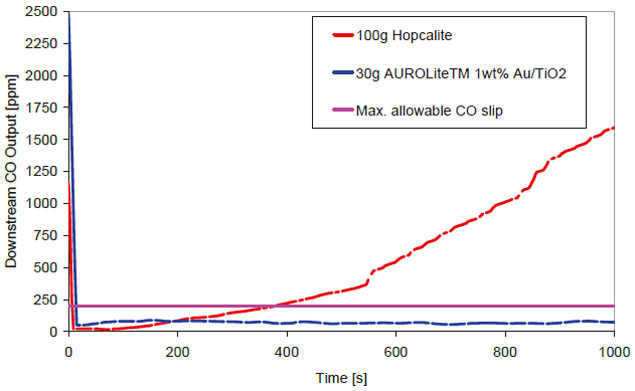
Figure 1 : EN403 Simulation, feed = 2500ppm CO in air, 90%RH, 30LPM, constant flow, bed = 4.7cm diameter, particle size 1 - 2mm.
The AUROliteTM Au/TiO2 catalyst has been used for the oxidation of CO and H2 by O2 and N2O by a group from the Technical University of Denmark, Lyngby [6]. This group also studied the low temperature oxidation of methane on AUROliteTM catalysts and found them active and stable with very little sintering up to 250°C [7]. The catalysts have also been shown to give complete conversion of propene at 250 – 350°C (i.e. VOC removal) and the gold particles do not sinter under these conditions either [8]. The alumina and zinc oxide catalysts give high conversions in methanol oxidation [9]. In addition, all three catalysts are currently being assessed for water-gas shift and selective hydrogenation activity.
Gas phase selectivity of gold catalysts has been confirmed in an unconventional PROX system, where the complete removal of CO from ‘dirty’ hydrogen (i.e. down to < 1ppm CO from up to 2000 ppm). For use in a fuel cell, the carbon monoxide still present in the hydrogen, obtained by water-gas shift or from other sources, must be removed to prevent it poisoning the platinum electro-catalyst inside the fuel cell. Gold catalysts have been found to be effective for this preferential oxidation reaction. Whereas PGM catalysts oxidize both the CO and the hydrogen, gold is selective for CO at close to ambient temperatures. This has enabled Project AuTEK to develop a new system for hydrogen purification for PEM fuel cells, trade named AUROPureH2TM based on the use of a 3wt%Au/TiO2catalyst used at room temperature [10].
3. Liquid Phase Reactions
Following their successful use in the oxidation of glucose to gluconic acid, the conversion of D-lactose to D-lactobionic acid (LBA) has been studied using Project AuTEK catalysts.
Results obtained using samples of the three AuTEK gold catalysts show that all of these materials produce high selectivity for LBA under mild conditions [11]. It has been found, however, that the activities vary with support type, with the 1wt%Au/ZnO catalyst being the most reactive, as indicated in Table 1:
Table 1 : Support effects in the oxidation of D-lactose to D-lactobionic acid
|
|
0.8%Au/Al2O3
|
1%Au/TiO2
|
1%Au/ZnO
|
|
M x t20%*
( gAumin)
|
9.12
|
7.4
|
5.6
|
|
Selectivity (%)
|
94.3
|
95.1
|
93.9
|
C
0=99.6 mmol l
-1, 0.2 g
cat, T = 60°C, pH=8, O
2=2.5ml min
-1, results adapted from ref 11.
*time in minutes to reach 20% conversion
AuTEK catalysts have also been used to catalyse the aerobic oxidation of aldehydes to esters, e.g. the conversion of acrolein tomethyl acrylate [12]:
Methyl acrylate is used in the manufacture of paints, in solvents and for acrylate coatings. This reaction proceeds in methanol using air as oxidant at room temperature, and therefore has the appeal of being 'green’ chemistry. The high conversions and selectivities are indicated in Table 2.
Table 2 : Synthesis of methyl acrylate from acrolein using 1wt%Au/TiO2 AuTEK catalyst
|
Time (h)
|
1
|
4
|
20
|
|
Conversion (%)
|
46
|
75
|
97
|
|
Selectivity (%)
|
43
|
66
|
87
|
Acrolein:Methanol 1:80, 0.3 mol % 1wt %Au/TiO
2, T = 25°C, results adapted from ref 12.
In addition, the use of AuTEK catalysts has been studied for the reaction between benzaldehyde and methanol to produce methyl benzoate [12], which has a pleasant odour and is used in perfumes. 100% conversion was obtained in ca. 20 min when the benzoic acid:methanol ratio was 1:30 in the presence of 0.2 mol% 1wt%Au/TiO2 AuTEK catalyst and 10mol% NaOMe at 40°C:
Other reaction types for which AUROliteTM catalysts are being investigated include glucose and glycerol oxidation, direct hydrogen peroxide synthesis and hydrodechlorination of water contaminants such as trichloroethene.
4.Conclusions
Gold catalysts are likely to make a large and unique contribution to applications where efficient chemical processes of the kinddescribed herein are required under mild energy-efficient conditions, as is adequately indicated by the examples obtained using AuTEK catalysts and related work described in recent literature.
References:
[1] G.C. Bond, C. Louis and D.T. Thompson, Catalysis by Gold, Imperial College Press, London, 2006.
[2] C.W. Corti, R.J. Holliday and D.T. Thompson, Topics Catal., 2007, 44, 331.
[3] D.T. Thompson, Nanotoday, 2007, 2, 40.
[4] Z. Ma, C. Liang, S.H. Overbury and S. Dai, J Catal, 2007, 252, 119.
[5] D.Ramdayal, J. McPherson, T. Khumalo, G. Pattrick and E. van der Lingen, Proc. GOLD 2009, Heidelberg, Germany, July 2009.
[6] G. Walther, D.J. Mowbray, T. Jiang, G. Jones, S. Jensen, U.J. Quaade and S. Horch, J. Catal, 2008, 260, 86.
[7] G.Walther, L. Cervera-Gontard, U. Quaade and S. Horch, Gold Bull, 2009, in press
[8] L.F. Liotta, G. Pantaleo, G. Di Carlo, A.M. Venezia, G. Dagenallo, M. Ousmane, A. Giroir-Fendler and L. Retailleau, Proc. 'Catalysis for Society’ Conference, Krakow, Poland, May 2008.
[9] M. Ziolek, Paper to be presented to 6th World Conference on Oxidation Chemistry, Lille, France, July 2009.
[10] J. Steyn, G. Pattrick, E. van der Lingen, M. Scurrell and D. Hildebrandt, South African Patent Appl., 2006, 1120.
[11] E.V. Murzina, A.V. Tokarev, K. Kordas, H. Karhu, J.-P. Mikkloa and D.Y. Murzin, Catal Today, 2008, 131, 385.
[12] C.Marsden, E.Taarning, D.Hansen, L.Johansen, S.K.Klitgaard, K.Egeblad , C.H.Christensen, Green Chem, 2008, 10, 168, and private communication.