|
|
(R)-(-)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane, min. 95% (R)-PHANEPHOS
C40H34P2; FW: 576.65; white solid; [α]D -62.6° (c 3.11, CH2Cl2); m.p. 222-225°
Note: Sold in collaboration with Chirotech for research purposes only. US Patent no. 5874629.
Technical Note:
1.See 15-0426.
|
100mg
500mg
|
|
|
(S)-(+)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane, min. 95% (S)-PHANEPHOS [192463-40-4]
C 40H 34P 2; FW: 576.65; white solid; [α]D +63.2° (c 3.27, CH 2Cl 2); m.p. 222-225°
Note: Sold in collaboration with Chirotech for research purposes only. US Patent no. 5874629. |
100mg
500mg
|
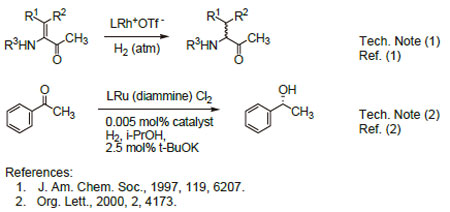
Technical Notes: 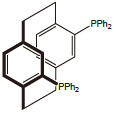 1.Highly enantioselective catalyst for the hydrogenation of dehydroamino acids, methyl esters under mild conditions.
2.Asymmetric hydrogenation of a wide variety of aromatic, heteroaromatic, and α-β unsaturated ketones.
 |
|
|
(R)-(-)-4,12-Bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane, min. 95% CTH-(R)-3,5-xylyl-PHANEPHOS
C48H50P2; FW: 688.86; white pwdr.
Note: Sold in collaboration with JM for research purposes only. US patent Application No 5874629 and patents arising therefrom.
Technical Notes:
1. See 15-0426.
2. See 15-0731.
|
100mg
500mg
|
|
|
(S)-(+)-4,12-Bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane, min. 93% CTH-(S)-3,5-xylyl-PHANEPHOS
C48H50P2; FW: 688.86; white pwdr.
Note: Sold in collaboration with JM for research purposes only. US patent Application No 5874629 and patents arising therefrom.
|
100mg
500mg
|
Technical Notes: 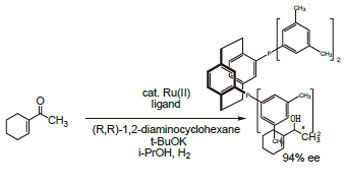
1. See 15-0426.
2. Chiral ligand employed in the enantioselective hydrogenation of various ketones.
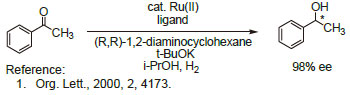 |
|
|
Dichloro[(R)-(-)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1S,2S)-(-)-1,2-diphenylethylene-diamine]ruthenium (II), min. 95% [325150-57-0]
RuCl 2[C 48H 50P 2][C 14H 16N 2]; FW: 1073.12; cream colored pwdr.air sensitive
Note: Sold in collaboration with Chirotech for research purposes only.US Patent nos. 5874629 and 6486337. |
10mg
50mg
250mg
|
Technical Note: 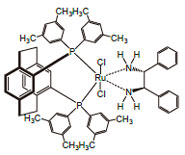
1. These Noyori catalysts containing the chiral xylyl-Phosphane ligands display exceptional activity and enantioselectivity in the asymmetric hydrogenation of a wide range of aromatic,heteroaromatic and α,β-unsaturated ketones. The reactions are performed under mild conditions, and substrate concentrations up to 40%w/v can be tolerated. Molar substrate/catalyst ratios of up to 100,000/l are achieved with excellent reactivity and enantioselectivity using commercial grade substrates and solvents.
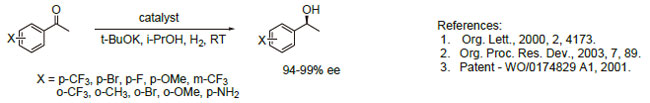 |
|
|
Dichloro[(S)-(+)-4,12-bis(di(3,5-xylyl)phosphino)-[2.2]-paracyclophane][(1R,2R)-(+)-1,2-diphenylethylene-diamine] ruthenium (II), min. 95%
RuCl2[C48H50P2][C14H16N2]; FW: 1073.12; cream colored pwdr. air sensitive
Note: Sold in collaboration with Chirotech for research purposes only. US Patent nos. 5874629 and 6486337.
|
10mg
50mg
250mg
|
Technical Notes:
1.The Noyori [(diphosphine) RuCl 2 (diamine)] catalysts containing the chiral ligand Xylyl-Phanephos display exceptionalactivity and enantioselectivity in the asymmetric hydrogenation of a wide range of aromatic, heteroaromatic and α,β-unsaturated ketones.
2.Reactions are performed under mild conditions at room temperature and typically at low H 2 pressures of 2-10 bar. Highsubstrate concentrations of up to 40% w/v are tolerated.
3.Molar substrate/catalyst ratios of up to 100,000/1 are achieved with excellent reactivity and enantioselectivity using commercial grade substrates and solvents.
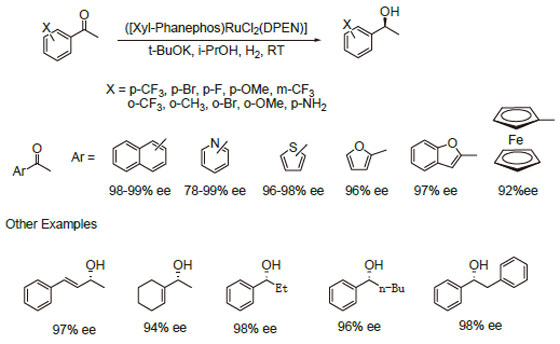
References:
1. Org. Lett., 2000, 2, 4173.
2. Burk, M.J.; Hems, W.; Zanotti-Gerosa, A. PCT WO/0174829 A1, 2001.
3. Org. Proc. Res. Dev., 2003, 7, 89. |