|
|
Buchwald Biaryl Phosphine Ligand Kit for Aromatic Carbon-Heteroatom Formation, Suzuki Coupling and Negishi Cross-coupling Contains the smallest unit size of the sixteen compounds listed 1 kit
|
|
|
racemic-2-Di-t-butylphosphino-1,1'-binaphthyl, 98% [ 255836-67-0] P; FW: 398.53; white xtl.; m.p. 147-149 |
250mg
1g
|
Technical Notes:
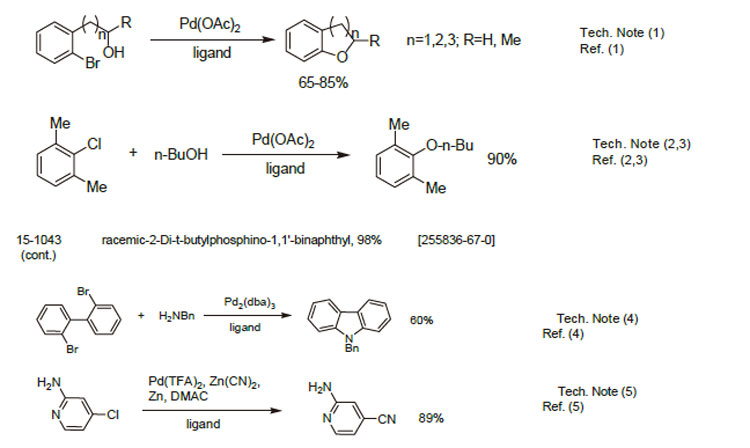
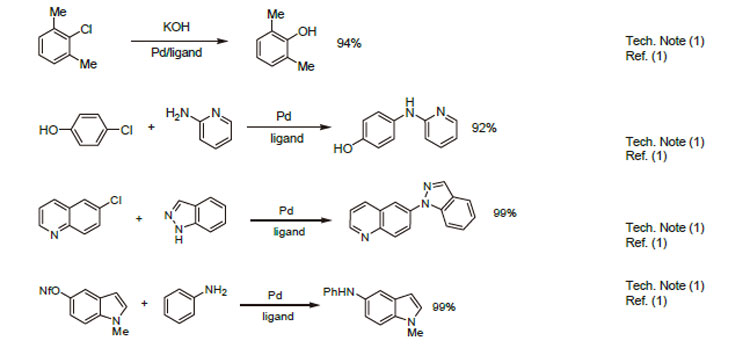
1. Ligand for the Pd-catalyzed formation of oxygen heterocycles.
2. Ligand for the intermolecular Pd-catalyzed synthesis of aryl ethers.
3. Ligand for the intramolecular Pd-catalyzed synthesis of aryl ethers.
4. Ligand for the synthesis of carbazoles by Pd-catalyzed double N-arylation reaction.
5. Ligand fo the Pd-catalyzed cyanation of (hetero)arylchlorides.
References:
1. J. Am. Chem. Soc., 2000, 122, 12907.
2. J. Am. Chem. Soc., 2001, 123, 10770.
3. J. Am. Chem. Soc., 2001, 123, 12202.
4, Tetrahedron, 2006, 62, 6792.
5. Org. Lett., 2007, 9, 1711. |
|
|
2-(Di-t-butylphosphino)biphenyl, 99% JohnPhos [ 224311-51-7] C 20H 27P; FW: 298.41; colorless xtl.; m.p. 85/ |
500mg 10g
2g 50g
|
Technical Notes:
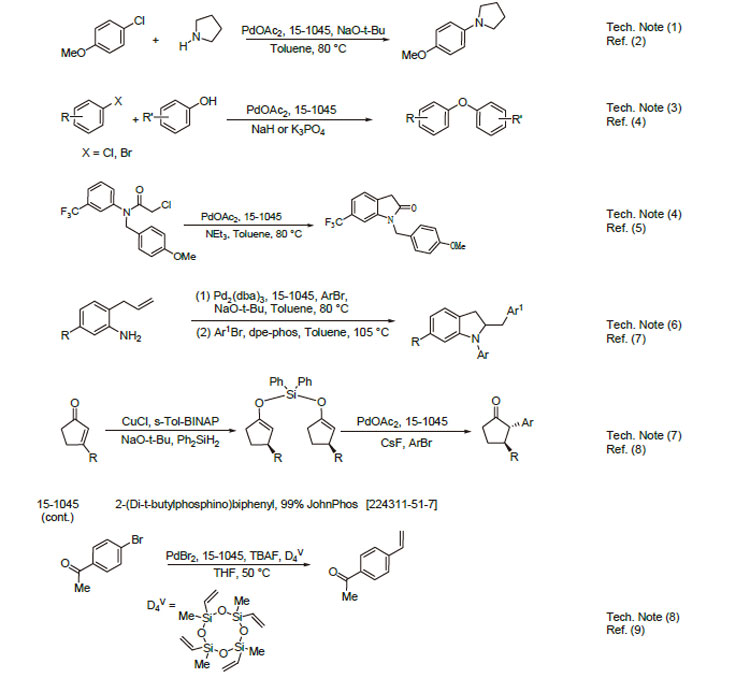
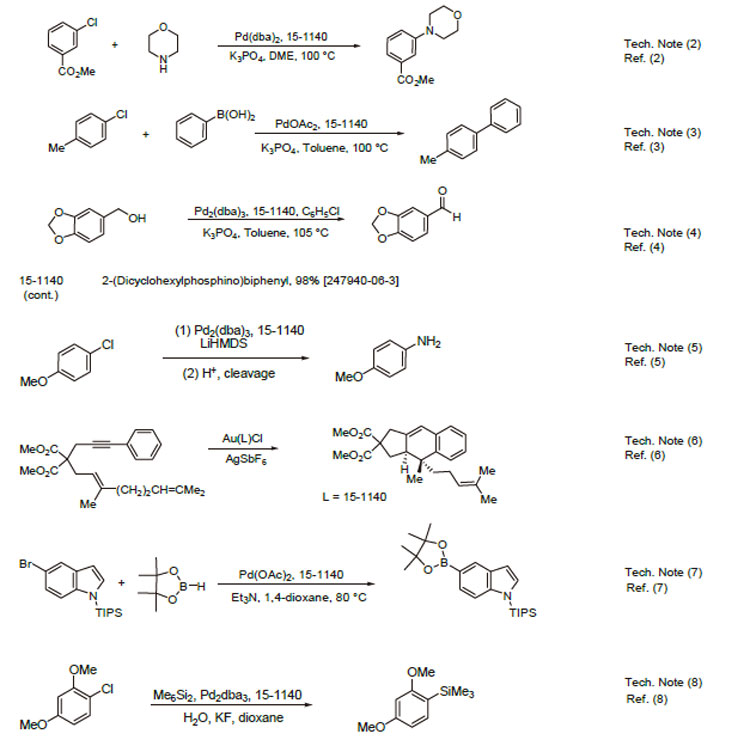
1. Ligand used in the palladium-catalyzed synthesis of aromatic amines from aryl chlorides, bromides and triflates.
2. Ligand employed in a very active and general catalyst for Suzuki coupling reactions using aryl chlorides,bromides and triflates.
3. Ligand for the palladium-catalyzed formation of diarylethers from aryl chlorides and bromides.
4. Ligand used in palladium-catalyzed synthesis of oxindoles from " -chloroacetanilides.
5. Effective ligand used in palladium-catalyzed arylation of thiazoles.
6. Used in the formation of 2-benzylindolines via sequential palladium-catalyzed N-arylation/cyclization/C-arylation.
7. Selective in the palladium-catalyzed arylation of silyl enol ethers formed from copper-catalyzed reduction of enones.
8. Ligand used in the palladium-catalyzed vinylation of aryl bromides.
9. Ligand used in the platinum-catalyzed synthesis of indolizinones.
10. Ligand used in the palladium-catalyzed diarylation of thiophenes.
11. Ligand used in the amination of vinyl halides by carbazates.
12. Ligand used in the regeioselective synthesis of 2,4-disubstituted syloles.
.jpg)
.jpg)
References:
1. Angew. Chem. Int. Ed., 1999, 38, 2413. 8. Org. Lett., 2004, 6, 4809.
2. J. Org. Chem., 2000, 65, 1158. 9. Org. Lett., 2006, 7, 63.
3. J. Am. Chem. Soc., 1999, 121, 9550. 10.Org. Lett., 2007, 9, 1169.
4. J. Am. Chem. Soc., 1999, 121, 4369. 11.J. Org. Chem., 2006, 71, 8309.
5. J. Am. Chem. Soc., 2003, 125, 12084. 12. Org. Lett., 2007, 9, 275.
6. Tetrahedron, 2003, 59, 5685. 13. J. Am. Chem. Soc., 2008, 130, 1526.
7. J. Am. Chem. Soc., 2004, 126, 13906. |
|
|
Di-t-butylphosphino-2'-(N,N-dimethylamino)biphenyl, 98% [ 224311-49-3]
(CH 3) 2NC 6H 4C 6H 4P(C 4H 9) 2; FW: 341.47; white xtl. |
500mg
2g
|
Technical Notes:
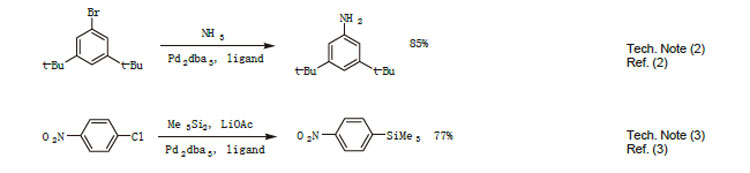
1. Useful ligand for Pd-catalyzed carbon-oxygen bond forming reactions.
2. Ligand used selective Pd-catalyzed arylation of ammonia.
3. Ligand used for selective Pd-catalyzed silylation of aryl chlorides.
References:
1. J. Am. Chem. Soc., 2001, 123, 12202.
2. J. Am. Chem. Soc., 2007, 129, 10354.
3. Org. Lett., 2007, 9, 3785. |
|
|
2-Di-t-butylphosphino-2'-methylbiphenyl, 99% [ 255837-19-5]
C 21H 29P; FW: 312.43; white xtl. |
Technical Notes:
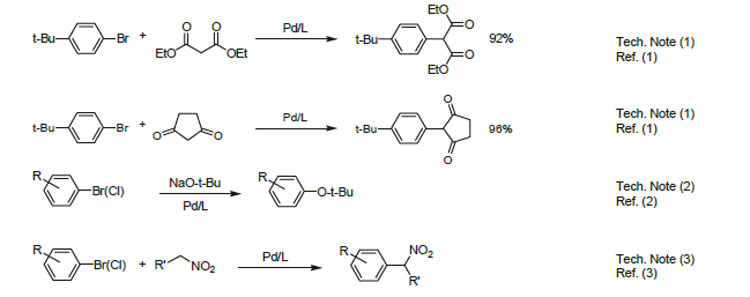
1. Ligand used in the Pd-catalyzed arylations of malonate esters and 1,3-diketones.
2. Ligand used in the Pd-catalyzed formation of t-butyl ethers from unactivated aryl halides.
3. Ligand used in the Pd-catalyzed " -arylations of nitroalkanes.
References:
1. J. Am. Chem. Soc., 2000, 122, 1360.
2. J. Org. Chem., 2001, 66, 2498.
3. J. Org. Chem., 2002, 67, 106. |
|
|
2-Di-t-butylphosphino-3,4,5,6-tetramethyl-2',4',6'-tri-i-propylbiphenyl, min. 98% [ 857356-94-6] C 33H 53P; FW: 480.75; white microxtl.; m.p. 166-168/ |
250mg
1g
|
Technical Notes:
1. Ligand for the palladium-catalyzed amidation of aryl chlorides.
2. Ligand for the palladium-catalyzed synthesis of phenols from aryl halides.
3. Ligand for the palladium-catalyzed coupling of aryl halides and secondary alcohols.
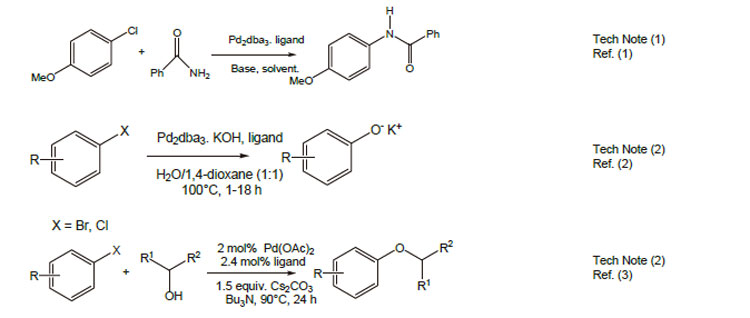
References:
1. J. Am. Chem. Soc., 2007, 129, 13001.
2. J. Am. Chem. Soc., 2006, 128, 10894.
3. J. Am. Chem. Soc., 2005, 127, 8146. |
|
|
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl, min. 98% t-butylXPhos [ 564483-19-8] C 29H 45P; FW: 424.64; white xtl.; m.p. 147-149/ |
500mg
2g
|
Technical Notes:
1. Ligand for the Pd-catalyzed synthesis of phenols and aromatic ethers.
2. Ligand for the Pd-catalyzed N-arylation of aminoheterocycles.
3. Ligand for the Pd-catalyzed N-arylation of indazoles and pyrazoles.
4. Ligand for the Pd-catalyzed amination of aryl nonaflates.
5. Ligand for the Pd-catalyzed stereoselective formation of alfa-O-glycosides.
6. See 15-1149 Technical Note 3.
References:
1. J. Am. Chem. Soc., 2006, 128, 10694. 3. J. Org. Chem., 2006, 71, 430.
2. Angew. Chem. Int. Ed., 2006, 45. 4.O rg. Lett., 2007, 9, 3173. |
|
|
2-(Dicyclohexylphosphino)biphenyl, 98% [ 247940-06-3] C 12H 9[P(C 6H 11) 2]; FW: 350.49; white xtl.; m.p. 103/ |
500mg 10g
2g 50g
|
Technical Notes:
1. See 15-1045.
2. Ligand used in the palladium-catalyzed sy nthesis of aromatic amines from aryl chlorides, bromides and triflates.
3. Ligand employed in Suzuki coupling reactions in volving aryl chlorides, bromides and triflates.
4. Useful ligand for the Pd-catal yzed oxidation of alcohols in the presence of chlorobenzenes.
5. Useful ligand for the Pd-catalyzed amination with ammonia equivalents.
6. Ligand for the gold(I)-catalyzed intramolecular [4+2] cycl oadditions involving 1,3-enynes and arylalkynes with alkenes.
7. Ligand used in the palladium-catalyz ed borylation of aryl bromdies.
8. Ligand used in the palladium-catalyz ed siliylation of aryl chlorides.
References:
1. Angew. Chem. Int. Ed., 1999, 38, 2413. 5. Org. Lett., 2001, 3, 3417.
2. J. Org. Chem., 2000, 65, 1158. 6. J. Am. Chem. Soc., 2005, 127, 6178.
3. J. Am. Chem. Soc., 1999, 121, 9550. 7. Helv. Chim. Acta., 2006, 89, 936.
4. Org. Lett., 2003, 5, 2485. 8. Org. Lett,, 2007, 9, 3785. |
|
|
2'-Dicyclohexylphosphino-2,6-dimethoxy-3-sulfonato-1,1'-biphenyl hydrate sodium salt, min. 98% C26H34NaO5PS.XH2O; FW: 512.58; light yellow solid
Note: Soluble version of 15-1143 S-Phos.
|
500mg
2g
|
Technical Note:
1. First general ligand for the Pd-catalyzed Suzuki-Miyaura coupling reaction of aryl chlorides and for the coupling of challenging substrate combinations in water.
.jpg) |
|
|
2-Dicyclohexylphosphino-2',6'-dimethoxy-1,1'-biphenyl, min. 98% S-Phos C26H35O2P; FW: 410.53; white xtl.; m.p. 158-162/
|
500mg
2g
|
Technical Notes:
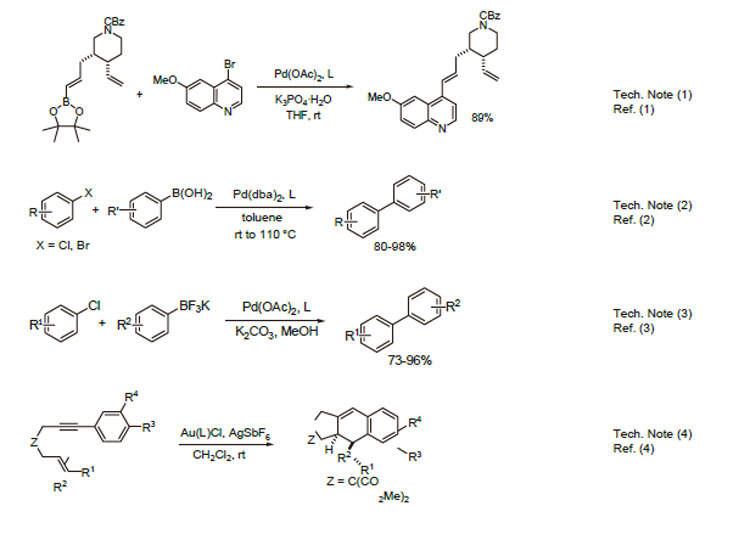
1. Ligand used in the palladium-catalyzed Suzuki-Miyaura coupling of boronic esters with heteroaryl halides.
2. Ligand used in the palladium-catalyzed Suzuki-Miyaura coupling of boronic acids with aryl halides
3. Suzuki-Miyaura coupling of potassium aryl trifluoroborates with aryl chloride.
4. Employed in the gold(I)-catalyzed intramolecular [4+2] cycloadditions of 1,3-enynes and arylalkynes with alkenes.
5. Effictive ligand in the palladium-catalyzed amination of heteroaryl halides.
6. Ligand employed in borylation of aryl chlorides.
7. Ligand used in palladium-catalyzed Kumada-Corriu cross coupling at low temperatures.
.jpg)
References:
1. J. Am. Chem. Soc., 2004, 126, 706.
2. Angew Chem. Int. Ed., 2004, 43, 1871.
3. Org. Lett., 2004, 6, 2649.
4. J. Am. Chem. Soc., 2005, 127, 6178.
5. Org. Lett., 2005, 7, 3965.
6. Angew. Chem. Int. Ed, 2007, 46, 5359.
7. J. Am. Chem. Soc., 2008, 129, 3844. |
|
|
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl, 98% DavePhos [ 213697-53-1]
(CH 3) 2NC 6H 4-C 6H 4P(C 6H 11) 2; FW: 393.55; white xtl.; m.p.115-119/ |
500mg
2g
|
Technical Notes:
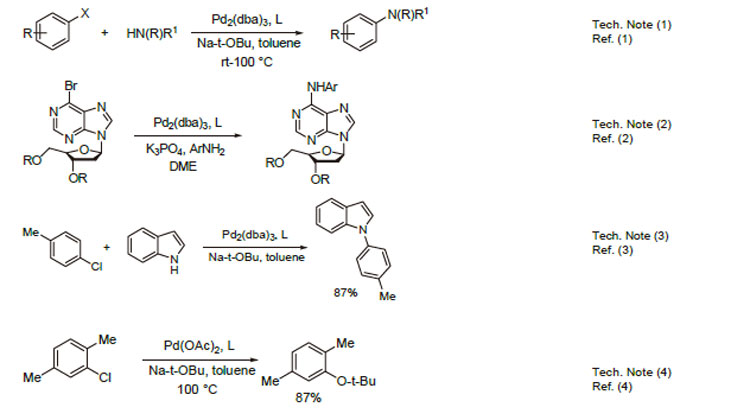
1. Ligand used in the palladium-catalyzed Suzuki coupling and amination of unactivated aryl chlorides. The reactions generally occur at room temperature and give high yields of product.
2. Ligand used in palladium-catalyzed C-N bond formation. A general synthesis of N6-aryl-2'-deoxyadenosine analogues.
3. Ligand used in palladium-catalyzed N-arylation of indoles.
4. Ligand used in palladium-catalyzed synthesis of aryl-tert-butyl ethers.
5. Effective ligand in the palladium-catalyzed arylation of ester enolates.
6. Ligand employed in arylation of ketone enolates using ortho-halo nitrobenzenes.
7. Ligand employed in the amination of aryl nonaflates using palladium catalysts.
8. Ligand used for cascade alkenyl amination/Heck reaction for the synthesis of indoles.
9. Ligand used in palladium-catalyzed Kumada-Corriu cross coupling at low temperatures.
10. Ligand used in rhodium-catalyzed intramolecular hydroamination of unactivated terminal and internal alkenes with primary and secondary amines.
.jpg) |
|
|
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl, 98% DavePhos [ 213697-53-1]
.jpg)
References:
1. J. Am. Chem. Soc., 1998, 120, 9722. 6. J. Am. Chem. Soc., 2002, 124, 15168.
2. J. Am. Chem. Soc., 1999, 121, 6090. 7. J. Org. Chem., 2003, 68, 9563.
3. Org. Lett., 2000, 2, 1403. 8. Chem. Eur. J., 2005, 11, 2276.
4. J. Org. Chem., 2001, 66, 2498. 9. J. Am. Chem. Soc., 2007, 129, 3844.
5. J. Am. Chem. Soc., 2001, 123, 7996. 10. J. Am. Chem. Soc., 2008, 130, 1570. |
|
|
2-Dicyclohexylphosphino-2',6'-di-i-propoxy-1,1'-biphenyl, min. 98% RuPhos [ 787618-22-8]
C 30H 43O 2P; FW: 466.64; white pwdr.; m.p. 123-124/ |
500mg
2g
|
Technical Notes:
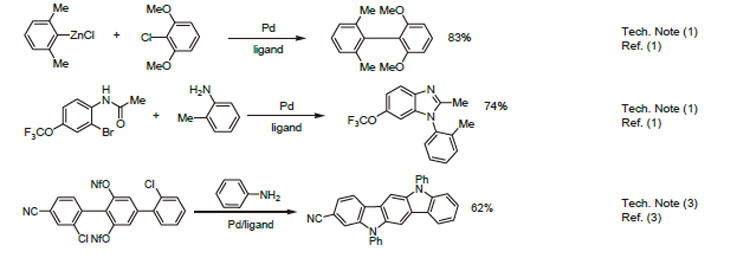
1. Ligand used for the Pd-catalyzed Negishi cross-coupling reaction of (hetero)arylchlorides.
2. Ligand used for the Pd-catalyzed synthesis of N-aryl benzimidazoles.
3. Ligand used for the Pd-catalyzed synthesis of heteroacenes.
References:
1. J. Am. Chem. Soc., 2004, 126, 13028.
2. Angew. Chem. Int. Ed., 2007, 46, 7509.
3. J. Org. Chem., 2007, 72, 5119. |
|
|
2'-Dicyclohexylphosphino-2,6-di-i-propyl-4-sulfonato-1,1'-biphenyl hydrate sodium salt [ 870245-84-4]
C 30H 42NaO 3PS; FW: 536.88; white solid |
500mg
2g
|
Technical Notes:
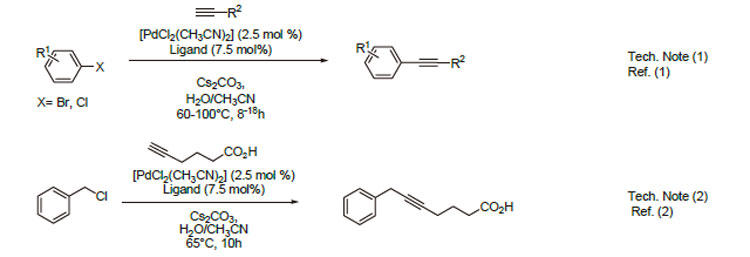
1. Water soluble catalyst for Sonogashira coupling reactions.
2. Water soluble catalyst for couplings of benzyl chlorides and terminal alkynes.
References:
1. Angew. Chem. Int. Ed., 2005, 44, 6173.
2. Synlett 2006, 2941. |
|
|
2-Dicyclohexylphosphino-2'-methylbiphenyl, min. 98% MePhos [ 251320-86-2]
C 25H 33P; FW: 364.51; white xtl.; m.p. 107-110/ |
500mg
2g
|
Technical Notes:
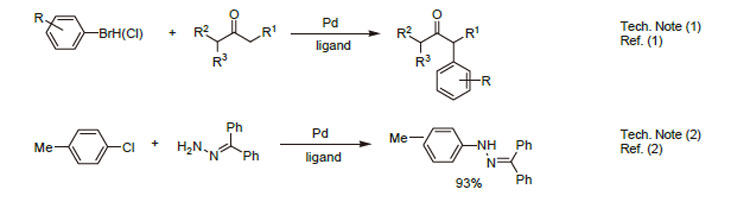
1. Ligand for the Pd-catalyzed formation of " -arylketones.
2. Ligand for the Pd-catalyzed amination reaction (See 15-1045).
3. Ligand for the Pd-catalyzed hydrazone arylation.
References:
1. J. Am. Chem. Soc., 2000, 122, 1360.
2. Adv. Synth. Catal., 2005, 347, 773. |
|
|
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl, min. 98% X-Phos [ 564483-18-7]
C 33H 49P; FW: 476.72; white pwdr,; m.p. 185/ |
500mg
2g
|
Technical Notes:
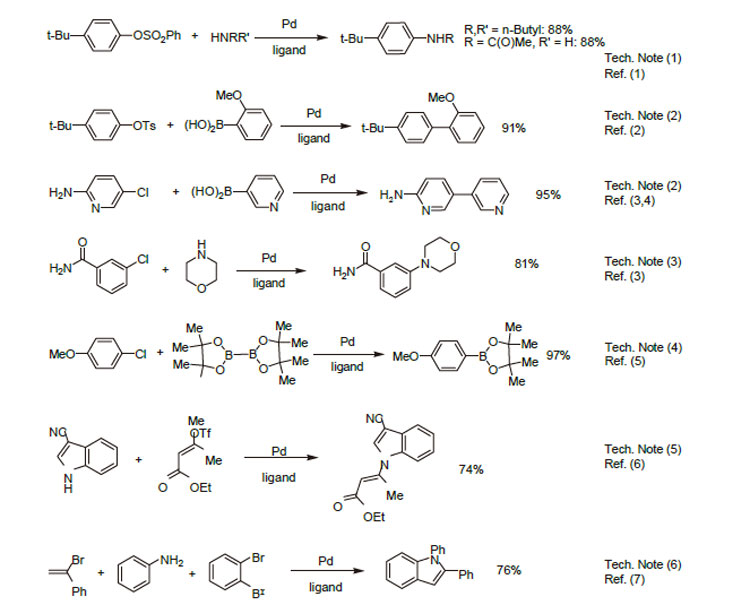
1. Exceptional ligand for Pd-catalyzed amination and amidation of aryl sulfonates.
2. Ligand used for the Pd-catalyzed Suzuki-Miyaura coupling reaction and carbonyl enolate coupling.
3. Ligand used for the chemoselective amination of arylchlorides.
4. Ligand used for the Pd-catalyzed borylation of aryl chlorides.
5. Ligand used for the Pd-catalyzed amination of vinyl halides and triflates.
6. Ligand used for the Pd-catalyzed three-component synthesis od indoles.
References:
1. J. Am. Chem. Soc., 2003, 125, 6653. 5. Angew. Chem. Int. Ed., 2007, 46, 5359.
2. J. Am. Chem. Soc., 2003, 125, 11818. 6. J. Org. Chem., 2005, 70, 8638.
3. Angew. Chem. Int. Ed., 2006, 45, 6523. 7. Angew. Chem. Int. Ed., 2007, 46, 1529.
4. J. Am. Chem. Soc., 2007, 129, 3358. |
|
|
2-(Dicyclohexylphosphino)-3,6-dimethoxy-2'-4'-6'-tri-i-propyl-1,1'-biphenyl, min. 98% BrettPhos
C35H53O2P; FW: 536.77; white xtl.; m.p. 191-193/
|
250mg
1g
|
Technical Notes:
1. Catalyst for cross-coupling reactions using aryl mesylates with electron-deficient anilines.
2. Catalyst for rapid C-N bond-forming process at low catalyst loading.
Reference:
1. J. Am. Chem. Soc., 2008, 130, 13552. |
|
|
2-Diphenylphosphino-2'-(N,N-dimethylamino)biphenyl, 98% [ 240417-00-9]
C 26H 24NP; FW: 381.46; white pwdr. |
500mg
2g
|
Technical Notes:
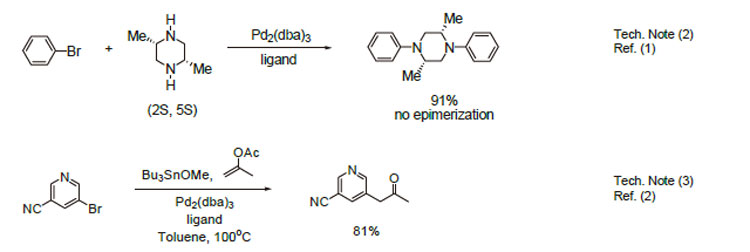
1. Useful ligand for sterically hindered substrates in the Pd-catalyzed amination reactions of aryl bromides.
2. Ligand employed in the coupling of enantiomerically pure cis-dimethylpiperazine with bromobenzene . Epimerization was not observed.
3. Ligand employed for the Pd-catalyzed heteroarylation of acetone.
References:
1. J. Am. Chem. Soc., 2001, 123, 1792.
2. Tetrahedron Lett., 2003, 44, 8869. |